To be clear, this article is not about quantum physics. Rather, it is about the bridge between classical physics and quantum physics, and how the former led to the latter. If you ever wondered where quantum physics came from, this is the place. Facts and Forces (coming soon) is helpful to understand a bit about classical mechanics, but is not essential. Relax, and enjoy the physics drama about to unfold before you.
We begin with a bit of history, because you can’t really understand the beauty of the human mind or the unparalleled power of scientific inquiry without it. In 9000 years people were convinced that the world was, inherently, logical. Cause and effect. Action and reaction. Isaac Newton solidified and vocalized these concepts with his laws of motion. He was the first to say out loud what everyone intuitively knew- that objects accelerated when you applied a force to them. Sounds simple enough but with this he was able to map out the trajectories of planets and the Earth itself. Among his many incredible triumphs was his work on gravity.
Newton mathematically identified the laws of classical physics, and set in motion our current age of explosive scientific growth. Especially his (and Leibniz’s) development of calculus- this would revolutionize mathematics well into the present day, where we rely on calculus from everything to the creation of microchips to the exploration of distant worlds.
In fact, everything that you assess the world with, all the physics that you consciously or unconsciously learned, is classical physics. When you drop something, it falls. When you push on something, it moves. But what every man, woman, and child grasps almost immediately is the concept of continuity.
If you tie a string to a marble, and start spinning it slowly, it travels a little farther from your hand. Spin it harder and it travels further away. It doesn’t leap from position to position, it moves gradually. That’s what continuity is, and it doesn’t just apply to position. If more air is pushed through a tuba, it will make a louder sound. Ease up on the airflow, and the sound gradually gets quieter. It doesn’t move in steps, it moves in slopes. It all seems obvious doesn’t it?
Well in the late 1800s and early 1900s, physicists found out that the world doesn’t actually work like that. Because energy doesn’t flow from one level to another, it jumps.
Basically, that marble swinging faster as you put more force into it? Illusions, Michael. It seems like that because it’s taking tiny, tiny steps. But there are steps that it just cannot take no matter how hard you try. The universe won’t let you. Which is totally at an odds with our concept of continuity.
So where is this all coming from? Well until 1900, people thought that Newton had figured it out. That everything else was just applying the laws of motion to smaller and smaller objects, until you could mathematically create or predict anything. Pierre Laplace, a rather arrogant yet brilliant mathematician, had this cheerful/hopeless thing to say:
“We may regard the present state of the universe as the effect of its past and the cause of its future. An intellect which at a certain moment would know all forces that set nature in motion, and all positions of all items of which nature is composed, if this intellect were also vast enough to submit these data to analysis, it would embrace in a single formula the movements of the greatest bodies of the universe and those of the tiniest atom; for such an intellect nothing would be uncertain and the future just like the past would be present before its eyes.”
Basically, he said that because of Newton’s law of action and reaction, everything in the world as it exists today is because of something that happened, and that ‘something’ was because of something that happened earlier. So the reason you’re feeling hungry is because molecules of pie were pushed by air currents to the sensory receptors in your nose, which sent a signal to your brain and reminded you that food existed. But those molecules of pie existed because your neighbor had decided to make a pie, because the day before he had seen pie ingredients on sale while grocery shopping. But those ingredients were on sale because the store had an excess of pie ingredients, because it’s been snowing a lot lately and people haven’t had time to make pie. So on, and so forth, until you get to the origin of the Universe which is the root cause of everything.
The problem with this view is that free will has no place. Everything that is going to happen is predetermined, and just because we can’t predict it now (because we don’t have all the data), doesn’t mean that we won’t ever be able to. Because if you can calculate the trajectory of every atom in the world, you can calculate exactly what is going to happen everywhere until the end of time, including human behavior.
This is what the world looked like. If you lived in between 1750 and 1900, you would have been convinced that the only thing left for humanity to do was to apply the laws of motion to everything. Powerful work, yes, but tedious. There wasn’t anything new to be discovered.
Until people started testing the limits of Newton’s laws.
For a little while people thought Newton was the end-all-be-all of science. But because of Thomas Young’s double slit experiment, people realized that Newton could be totally, completely, hilariously wrong. I go into the light fiasco in Losers of Light (coming soon), but the point is that people thought that maybe we shouldn’t take Newton’s word as gospel truth (this is accompanied by people saying we shouldn’t take gospel truth as gospel truth, but I digress). So they started using his laws of motion and applying them to extreme cases, to see if they held up to the most brutal of tests.
Most of the time, they passed. Newton’s laws are not trifle, tenuous things. As much as I dissed Newton, his three laws are phenomenally brilliant. And people didn’t think they were wrong- they just wanted to make sure. Most of the scientists conducting these tests were sure that they would hold up.
Then one day, they didn’t.
In 1860, Gustav Kirchoff started looking at black-body radiation. A black body is exactly what it sounds like- something that is completely, totally black. As in, it does not reflect any light. Taking a few steps backwards, the colour of something is due to the wavelengths of light that it reflects. So a blue object would absorb red, orange, yellow, green, indigo, and violet light, but reflect blue, and that’s what hits our eyes. A black object absorbs all light, and nothing hits our eyes. Black is the absence of light, hence why a dark room is black.
A black body can emit light, however, when it heats up. For example, when you heat up a piece of coal, it glows a dim red. If you heated it up more, it would glow orange, then yellow, then white, then blue. Kirchoff proved that the only thing that determined the colour of a black body was how hot it was. Nothing else. Not even the type of material- a black piece of coal would glow the EXACT same colour as a black piece of metal, or any other black body, as long as they were the same temperature.
This makes black bodies very easy to study. So Kirchoff found out the red-orange-yellow-white-blue transition, and matched each of the colours to a specific temperature. He did this by heating up a black body and measuring the emitted light. Pretty simple stuff.
But according to Newton, you should be able to calculate those colours, right? There should be an equation telling you “if the temperature is ___ then the colour is __.” Kirchoff proved beyond a doubt that such an equation existed. And every single physics equation up till that point was derived from Newton’s laws- because in a hundred and fifty years, people were finding that they applied to EVERYTHING. But Kirchoff could not for the life of him find the connection between Newton’s laws and the colour spectrum of a heated black body.
So he challenged physicists around the world to find that connection. Now to be clear, he still thought that there was a connection. He just thought that he couldn’t figure it out, and that it was a difficult formula. A challenge that he couldn’t keep to himself- he had to share it with other physics geeks.
Physicists took him up on his challenge and worked to find the equation that could calculate the colour of a black body when you knew its temperature. (By the way, this also allows you to tell how hot a black body is from its colour- quite useful for determining how hot, say, a distant star is).
40 years later, and still, no one could figure it out. But physicists were catching onto something weird. If you followed Newton’s laws to their conclusion, you just don’t get the colour spectrum that Kirchoff found.
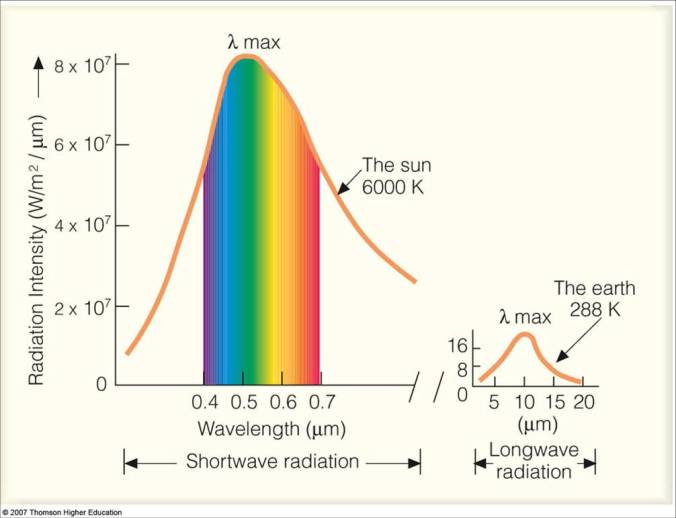
To understand what that means, we need to understand what a black body curve is. Basically, when something glows hot, it emits light of many different wavelengths. Take the sun, for example. We know that it shines really bright and emits light of all colours, including the ones we can’t see- it emits all the light of the visible spectrum, plus infrared light, plus ultraviolet and gamma radiation. The above curve is how much of each wavelength is emitted. As you can see, most of the light is visible light, peaking at green. Now if you were to mix all the colours in the exact amounts that you see above, you would get white light with a tint of yellow (exactly the colour of the sun). But we know that it contains all the colours- Newton discovered this earlier when he shone sunlight through a prism and got the rainbow seen above. If you measured the intensity of each of those colours, you would get the almost exact values of the above graph. Green would have the highest intensity, at 8×10^7 W/m^2. The red and violet strips would be a little over 5×10^7. No matter what.
This is what Kirchoff discovered. But he could not calculate it. There was no mathematical explanation for why black bodies did that. In fact, according to Newton’s laws, this is what the curve should look like (the dotted line).
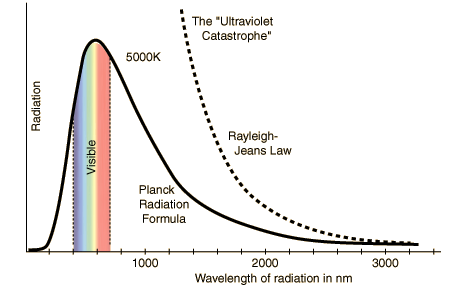
According to Newton’s Laws, a black body should emit mostly ultraviolet radiation. As in, the sun should crisp you like a newspaper in a bonfire
Most of the sun’s energy would take the form of ultraviolet and gamma radiation. Gamma radiation doesn’t turn you into an angry green monster that smashes things. That much gamma radiation would turn you into a pile of ash. Instantly. No life could exist on Earth because the sun would scorch it. There’s a reason why we wear sunscreen- even the low levels of ultraviolet radiation shown on the actual curve (the amount of ultraviolet radiation is the level of the graph just to the left of violet) are enough to cause serious burns. If the sun emitted any more of it, life wouldn’t have had a chance to evolve.
Why Newton’s laws always lead you to that conclusion is a bit technical, and if you’re not comfortable or just don’t want to know, you can skip the section in red. But if you’re burning to know why Newton’s laws show that the sun should be an enormous death ray, I encourage you to read it. It’s some pretty nifty stuff.
According to the laws of motion, black bodies glow like this: energy causes the individual molecules to vibrate. Vibration of molecules is the definition of heat– when you compare a glass of hot water to a glass of cold water, the only difference is that the molecules of hot water are vibrating faster than the molecules of cold water. To be clear, it isn’t Newton’s laws of motion that directly lead to this conclusion- James Clerk Maxwell’s equations of electromagnetism were used, which are based off of Newtonian physics.
So a black body eventually gets so hot ie vibrates so quickly that the frequency approaches that of light. For reference, the frequency of infrared radiation is about 10^12 Hz- vibrating at 1000000000000 (1 followed by 12 zeroes) per second. That’s on the slow end- ultraviolet radiation is about 10^16 Hz. Those extra four zeroes mean that it vibrates 10000 times faster than infrared radiation. So the idea that once something gets hot enough that it starts vibrating at those speeds, it starts emitting light as well as heat (remember, we feel infrared radiation as heat! that’s what ‘heat vision’ is), is actually something that makes a ton of sense.
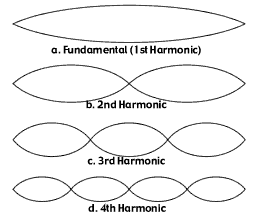
But there are another set of principles which apply to vibrating objects, or harmonic oscillators, and when you consider them (you have to), Take a violin string. It is possible for that string to vibrate with different wavelengths, creating different harmonics in the case of music.
Anything that vibrates will be able to vibrate in different modes. Any of these modes are equally likely, so physicists asked themselves this: what happens in the case of the vibrating atoms in a black body?
The higher modes are going to produce light which a much, much shorter wavelength. Basically, the atoms that happen to vibrate at the 4th harmonic will produce ultraviolet rays, while the atoms on the 1st harmonic will produce infrared or red rays. And when you consider all the atoms in a black body, the ones that produce ultraviolet rays will be equal in number to those producing infrared rays.
But, as we mentioned earlier, ultraviolet rays have a much higher amount of energy per ray than infrared rays! So even though the number of rays in each wavelength is about the same, most of the energy will take the form of ultraviolet rays. It’s analogous to wealth distribution: if you take a hundred people with different amounts of wealth, and ask each of them to build a building, they won’t be equal in size- the person with a billion dollars will build a skyscraper while the person with a dollar will build a toothpick/marshmallow tower. And what matters is the amount of material in each building- not the person that built it. In the same case, it’s not the number of atoms that produce ultraviolet rays that matters- it’s the fact that when you look at how the energy is distributed, most of it will take the form of ultraviolet rays.
So in the case of the sun, which burns at around 5000 Kelvin, most of that energy is going to take the form of ultraviolet rays. And that obviously doesn’t happen- because for one, we don’t actually measure that much ultraviolet radiation from the sun, and for another, even that tiny amount of ultraviolet radiation that we do measure is enough to cause serious problems, like burns and skin cancer. So all the ideas of atoms vibrating to produce electromagnetic waves? They HAVE to be garbage. But no one could understand why.
Then, in 1900 Max Planck did it. He found an equation that exactly predicted the emitted light based on temperature. But what he did was bizarre, and as he himself called it, a ‘technical fix’. He assumed that instead of energy being continuous, it must be transmitted in tiny little chunks, or packets.
Everyone in the physics community gave a collective Huh?! when they saw his equation. The idea that energy wasn’t continuous was even more ridiculous to them than it is to you. Because yes, what he said was that when you throw a ball, you can throw it fast, you can throw it slow, but there are some speeds in between that are impossible to throw at.
Even Planck thought that there had to be a mistake. He thought that someone would find a better solution, one where they didn’t have to assume that unicorns existed in order to make the right predictions. But it took a man we all know and love to realize that it wasn’t a mistake. Planck had caught on to something that turned the world of physics on its ear, and shattered our perceptions of a logical, predictable universe. It was Albert Einstein that suggested that energy actually does transfer in chunks.
He wasn’t looking at blackbody radiation. He was looking at the photoelectric effect- when you shine a light on a metal, it starts throwing electrons everywhere. The problem is, according to classical physics, there should be a time delay- the dimmer the light, the longer it takes for the metal to absorb the energy, then eject electrons. But if energy did indeed travel in packets, then there would be no time delay. Einstein remembered 5 years earlier that Max Planck had solved the black body radiation problem by assuming the same thing. He knew that it couldn’t be just a coincidence, and from then on physicists knew that energy behaved totally differently than Isaac Newton and every physicist since had believed.
For that, he got the Nobel Prize in 1921. Max Planck was also awarded the Nobel Prize, in 1918. These were heavily deserved prizes, because from that weird idea, birthed an unprecedented revolution in physics. Thus began the quantum era, where we learned that the world wasn’t ordered, predictable, and stable. It was rife with random processes and contradictions, and sturdy-sounding ideas such as ‘Newton’s Law of Universal Gravitation’ gave way to shaky-sounding ones, such as ‘Heisenberg’s Uncertainty Principle’. How is it even possible to have a physics theorem based on uncertainty? Isn’t physics about being certain about how the world works? But that’s exactly what the quantum revolution was- it turned our world upside down, or rather, made us realize that it was never right side up in the first place.
The small amount of uncertainty, or randomness, could have huge consequences for the nature of reality. Take the pie example I illustrated earlier. (The reason you’re feeling hungry is because molecules of pie were pushed by air currents to the sensory receptors in your nose, which sent a signal to your brain and reminded you that food existed. But those molecules of pie existed because your neighbor had decided to make a pie, because the day before he had seen pie ingredients on sale while grocery shopping. But those ingredients were on sale because the store had an excess of pie ingredients, because it’s been snowing a lot lately and people haven’t had time to make pie. So on, and so forth, until you get to the origin of the Universe, which is the root cause of everything.)
What if one atom did not collide with another, because one electron was not in precisely the right place, and that even though 99.999% of the time it is, this time it just wasn’t? Then, because the collision was delayed, the snowstorm didn’t start until after the clouds had passed the town, and people made pie at a regular rate. And then your neighbour would not have decided to make the pie, and as a result you don’t get hungry until an hour later, and that completely changes your day? What if instead of going immediately to lunch, you went to the library and met your future spouse? This is called the butterfly effect- the idea that tiny changes have a huge effect on the overall outcome. And these tiny, random, unpredictable changes occur all the time, making it truly impossible to predict the future with perfect accuracy.
For the consequences of quantum mechanics and an understanding of just how weird our world is, read on to Major Queries of Quantum Mechanics.
Sources:
Image of sun’s black body curve retrieved from http://apollo.lsc.vsc.edu/classes/met130/notes/chapter2/plank_e_sun.html
Image of ultraviolet catastrophe retrieved from http://hyperphysics.phy-astr.gsu.edu/hbase/mod6.html
Image of harmonics retrieved from http://www.noyceguitars.com/images/technotes/articles/harmonic.gif
Pingback: Majorana’s Mask | Surreal Science Stuff